SaMD or Software as a Medical Device can be described as a software constructed to be used in medical devices. These softwares can be run on different operating systems and virtual platforms. Examples of SaMD include:
- standalone softwares running on general computers, smartphones and tablets.
- SaMD that can detect interrupted breathing during sleep by using a microphone of a smart device.
- SaMD that can analyze the heart beat rate.
A software can behave as SaMD when it is run on a medical hardware device, but does not serve any intended purpose for the hardware. However, if the software controls the hardware device or drives it, then it cannot be called SaMD.
Softwares, when used as medical devices have several challenges, the major ones being clinical evaluation, scientific validity and clinical validation. All these three must be performed with accuracy as they are crucial for a successful launch of SaMD in the clinical market.
- Clinical evaluation of SaMD is described as a set of ongoing activities which are conducted to assess and analyze the SaMD’s safety, performance and effectiveness.
- Scientific validity/ Valid Clinical Association of SaMD refers to the extent of clinical acceptance of the SaMD’s output in terms of concept, conclusion and measurements. It ensures that the output of SaMD corresponds accurately to the healthcare situations and conditions in the real world.
- Clinical validation can be described as the relationship between verification and validation results of the algorithm used and the clincial condition in question. Clinical validity is determined by the manufacturer of the SaMD while developing it before distribution (pre-market) and when it is in use after distribution (post-market).
The basic programming model of a SaMD is given below.
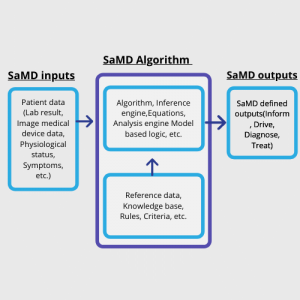
Figure 1: SaMD Basic Programming Model
Different softwares are used for medical purposes, and they include the following:
- Advanced analytics – described as a device which can use big and large complex data sets from a variety of sources by identifying and analyzing the data, and uses this data for medical devices.
- Artificial intelligence – described as a device which mimics human learning and reasoning. Machine learning, neural networks and natural language processing are all different types of AI.
- Cloud – described as a device which has an internet based computing system, whcih provides with computer processing resources and data. Several resources such as servers, computer networks and storage devices can share the pool of data from the cloud system.
- Cybersecurity – described as a device which prevents unauthorized access, misuse, modification, or denial of use of any information that can be transferred to an external recipient from a medical device.
- Interoperability – a device that exchanges and uses information with another medical/non-medical product via an electronic interface.
- Medical Device Data System (MDDS) – a software (or hardware) used to transfer and store data, convert formats of data and display data of the medical device without the alteration of parameters of any other connected medical device.
Premarket notification is a key element for the regulation of medical devices. There are diverse medical products in the market such as glassware and reagents of laboratories, spectacles, lenses, etc, which are not true medical devices. The system of identifying and classifying generic devices is crucial for a successful regulation of such products. To define safety and effectiveness of all types of medical devices, the factors taken into consideration are:
- patient population in question
- labelling and advertising of the ways to use a medical devise
- balance between benefits and risks to health when using a medical device
- reliability of the medical device
The International Medical Device Regulator’s Forum (IMDRF) seeks to demonstrate safety, effectiveness and performance of SaMD. It suggests that a software used in medical sciences is unique with respect to its connectivity. The manufacturers of softwares related to medical devices are encouraged to use this feature of uniqueness to modify the softwares to suit the real-world performances.
It is important to ensure safety in the new technologies used for medical devices, and the way it is done is illustrated below:
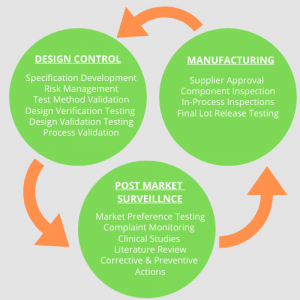
Figure 2: Good quality systems ensure safety of patients
Several opportunities associated with software driven medical sciences are discussed below.
- Techniques of advanced analytics can be used to analyze datasets which normally cannot be analyzed by humans. These include big and complex data sets that can only be analyzed by specialized softwares.
- Advanced analytics can discover new patterns in data.
- A patient’s melanoma can be analyzed by an imaging system through comparison with a repository of past melanoma data such as images, diagnosis and treatment plans.
- A personalised 3D model of the coronary arteries can be created from a standard CT by a software, and the model can predict the impact of blockages on flow of blood through the arteries.
- Algorithms used in imaging systems of artificial intelligence can provide diagnostic information on skin cancer.
- AI used in a smart ECG device can estimate the probable occurrence of acute cardiac ischemia (ACI)
- A mobile colposcope can use images stored in cloud and retrieve it for use in the doctor’s chamber later on.
- Cardiovascular images acquired from magnetic resonance scanners can be analyzed by a software through a cloud-based picture archiving and communication system.
- Data of patients from a pulse oximeter can be used by a designed infusion pump to change the settings of another infusion pump as per requirement.
- A software can be used to adjust the pressure, flow settings and volume of air of a ventilator, and also to collect output data about a patient’s carbon dioxide level.
- Patient data from several devices can be received by a centralized patient monitoring system.
- medical data can be electronically displayed
Given below is the total product life cycle of a medical device – the science cycle and the regulatory cycle:

Figure 3a: Science cycle

Figure 3b: Regulatory Cycle
References:
- https://www.fda.gov/medical-devices/digital-health/digital-health-criteria
- http://www.imdrf.org/docs/imdrf/final/technical/imdrf-tech-170921-samd-n41-clinical-evaluation_1.pdf
- https://www.ncbi.nlm.nih.gov/books/NBK209791/pdf/Bookshelf_NBK209791.pdf
- Kramer DB, Tan YT, Sato C, Kesselheim AS. Ensuring medical device effectiveness and safety: a cross–national comparison of approaches to regulation. Food Drug Law J. 2014;69(1):1-23, i. PMID: 24772683; PMCID: PMC4091615









