In brief
Manufacturers perform post-market surveillance to gather and assess experience obtained from medical devices that have been placed on the market and determine the need for any action. Post-market surveillance is an important tool for ensuring that medical devices remain safe and effective and taking action if the hazard of continuing to use the device outweighs the benefit. The examination of post-market monitoring experiences can also reveal areas where the medical device could be improved.
Introduction
Like many other worldwide regulatory frameworks, the WHO Global Model Regulatory Framework for Medical Devices, which includes in vitro diagnostic medical devices, necessitates the deployment of post-market surveillance systems. Receiving and assessing comments are the bare minimums for post-market monitoring, but this may be broadened to include other tasks. The operations of national regulatory authorities (NRAs) acting in response to reports of adverse events received, known as vigilance, are also included in the WHO Global Model Regulatory Framework for Medical Devices. The terms “adverse event” and “incident” may be used interchangeably in various jurisdictions. The word “incident” will be used in this advice to refer to a variety of experiences that may be obtained when using a medical device.
The WHO Global Model Regulatory Framework for Medical Devices defines post-market surveillance as the activity of NRAs. Market monitoring is the term used in this paper to describe the operations carried out by NRAs. The phrase “post-market monitoring” is only used in this text to refer to processes carried out by manufacturers. As a result, post-market surveillance, vigilance, and market surveillance are all interchangeable concepts. Users’ insights about the use of medical devices are shared with manufacturers. Manufacturers notify NRAs of specific instances and inform them of the steps taken. The NRA will look into the manufacturers’ inquiries and any further steps. This has to do with NRAs’ market surveillance obligations. The term “market surveillance” refers to the entire set of operations carried out.
Basic principles of post-market surveillance
Manufacturers of medical devices undertake pre-market evaluations of product quality, safety, and performance before releasing them into the market. Risk management concepts are used to decide risk reduction and residual risk tolerance. However, problems may develop once the medical equipment is on the market.
Manufacturers’ responsibilities
Even though medical devices are conceived, developed, manufactured, and distributed worldwide following extensive pre-market testing, residual concerns regarding safety and performance will persist throughout the product’s lifetime. This is attributable to various reasons, including intrinsic product variability, factors impacting the medical device’s use, environment, human interaction, and medical device failure or overuse. Before a medical device is released on the market, design and development processes guarantee that the remaining risks are acceptable compared to the anticipated benefits.
Actions of users about manufacturers’ post-market surveillance
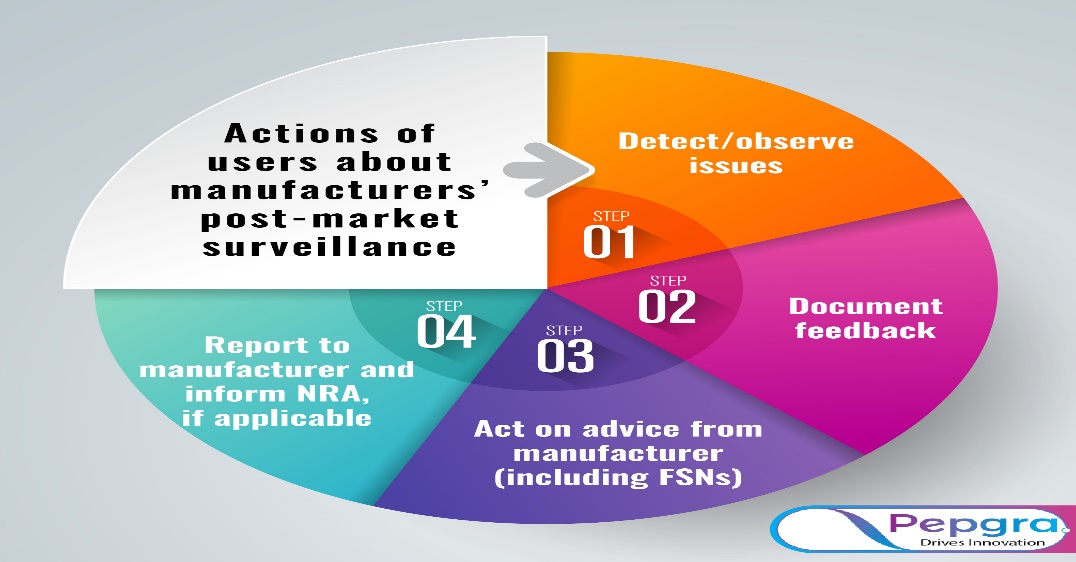
Post-market surveillance mechanisms
The information that can/will be gathered during post-market surveillance is dependent on the information that can/will be collected. The manufacturer must first determine the post-market surveillance objectives for any given medical device or collection of medical devices, and the maker must next decide which sources are required to achieve these goals. Data will be grouped and analysed based on this.
Electronic post-market surveillance system for medical devices
The gathering of information concerning adverse occurrences using medical devices and communicating such information among Health Care Institutions and Manufacturers Competent Authorities is a fundamental problem in the health care industry. An Electronic Post Market Surveillance System that solves the problem above effectively and securely while adhering to international standards. The PMS Server Application is deployed on the premises of the manufacturers/suppliers, while the PMS Client Application is installed at the Health Care Institutions. The PMS programmes are used to manage PMS Reports and Responses, and they include significant characteristics, including user-friendly interfaces, interoperability, and various implementations based on performance and cost requirements. The system was assessed methodically.
Conclusion
The goals and methods for post-market monitoring for medical devices undertaken by manufacturers with the help of their economic operators, market surveillance conducted by regulators, and the participation of other stakeholders in these processes are discussed in this paper. It explains the steps required after a medical device is placed on the market to ensure that it continues to meet the standards for safety, quality, and performance.
About Pepgra
Pepgra has a lot of post-market surveillance (PMS) report writing knowledge. The European Medicines Agency (EMA), the Food and Drug Administration (FDA), and other local nation criteria are followed by our PMS specialists. Pepgra CRO contributes to the advancement of public health by detecting and assessing safety indications from available data sources using evidence-based techniques and then recommending appropriate regulatory actions such as labelling changes, Risk Evaluation and Mitigation Strategies (REMS) communication of relevant safety information. Pepgra CRO, with its extensive understanding of the pharmaceutical business and remarkable in-house skills, provides high-quality and cost-effective post-marketing monitoring services to its clients.
References
- Chaudhry, Junaid, et al. “Enabling Technologies for Post Market Surveillance of Medical Devices.” (2018).
- Vlachos, I., Dimitris Kalivas, and Ourania Panou-Diamandi. “An electronic post-market surveillance system for medical devices.” Computer methods and programs in biomedicine2 (2003): 129-140.






