In brief:
- The major steps in conducting a clinical trial study are study design, study conduct, data analysis and reporting of the findings.
- Randomized clinical trials are deemed as a gold standard method for analyzing and evaluating the safety and effectiveness of medical devices or pharmaceutical drugs.
- The most challenging part of conducting a randomized clinical trial are related to handling ethical and regulatory systems.
Clinical study trial is majorly comprising of four components which include study design, the conduct of the clinical trial, analysis of acquired data and reporting the results. In this article, we will discuss the details of the major components and the challenges that come at every stage and finally, the limitations and future prospects.
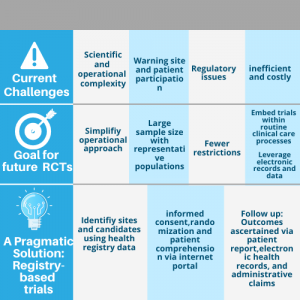
Randomized clinical trial (RCT) is considered as the gold standard method to understand the medical interventions by evaluating the effectiveness of the treatment and the safety of the patients or participants. In clinical research, studies like RCT are known for the evidence-based decision making, and a well-constructed clinical trial study will provide with the most reliable data with the effectiveness of clinical interventions. Although, RCT is the reliable method for measuring the effectiveness, the quality of these studies has always been considered because of its multidisciplinary nature and the multistep process as they face different challenges that vary among countries.
The basic principle in conducting clinical research is that the data collection must be keenly monitored as it is the major source of information that will later be analyzed. Such monitoring helps in avoiding the bias later and ensures the risk of the patients or participants present in the clinical trial study. This can also be mentioned as a challenging section in conducting a clinical study, and many institutions conducting the study have less awareness of the research priorities and conducting the study in developing countries often cause infrastructural barriers, ethical and cultural issues, organizational problems, etc.
The analysis of randomized clinical trials can be misleading by the timeliness or the poor design of study design and misinterpretation of well-designed randomized clinical trials. Besides, several limitations such as physician competence, faults in the patient selection process, applicability, randomization, and the populations used for study can also affect the effectiveness of the clinical study and produce misleading results.
The major challenges in conducting randomized clinical studies are associated with patient recruitment suitable for the clinical research, barriers in handling ethical concerns and regulatory systems, lack of skilled support staffs and experienced clinical investigators, lack of infrastructure, awareness and motivation and lack of time for conducting a clinical trial study, and these challenges can be avoided using a complete well-driven planning, proper design of the study and support from a contract research organization.
On the future prospect, the randomized clinical trial must adapt to the situation and evolve in responding to the environmental changes so as to conduct a high-quality clinical trial study. In addition, a collaborative effort will also drive the clinical research in the right direction, and by reconsidering the existing challenges, a clinical trial study should arise with innovation from utilizing the shared patient data to drive the development of medical technologies and pharmaceutical drugs for the human use. However, the obtained clinical trial data should be protected with robust technologies, and individual privacy must also be considered.
Reference:
- Fatemeh Varse, Leila Janani, Yousef Moradi, Masoud Solaymani-Dodaran, Hamid Reza Baradaran, and Shahnaz Rimaz, Challenges in the design, conduct, analysis, and reporting in randomized clinical trial studies: A systematic review, Med J Islam Repub Iran. 2019; 33: 37.
- London L, Hurtado-de-Mendoza A, Song M, Nagirimadugu A, Luta G, Sheppard VB. Motivators and barriers to Latinas’ participation in clinical trials: the role of contextual factors. Contemporary clinical trials. 2015;40:74–80.
- McKenzie JE, Herbison GP, Roth P, Paul C. Obstacles to researching the researchers: A case study of the ethical challenges of undertaking methodological research investigating the reporting of randomized controlled trials. Trials. 2010;11(1):28.
- Hans-Georg Eichler, Fergus Sweeney, The evolution of clinical trials: Can we address the challenges of the future?, Volume: 15 issues: 1_suppl, page(s): 27-32
- SchuylerJonesMD et al., The Changing Landscape of Randomized Clinical Trials in Cardiovascular Disease, Journal of the American College of Cardiology, Volume 68, Issue 17, 25 October 2016, Pages 1898-1907.






