CRO Biostatistical Services
Services
- Clinical Trial Audit & Monitoring
- Clinical Study Design
- Global Regulatory & Clinical Writing
- Clinical Biostatistics
- Clinical Trial Patient Recruitment
- Regulatory Affairs
- Clinical Data Management
- Post-Market Surveillance
- Clinical Technology & Process
- Healthcare Analytics
- Global and Local Literature Search Screening
Clinical Biostatistics & Statistical Programming
Pepgra’s biostatisticians direct sponsors in trial design; conduct analysis and evaluation of clinical trials—on par with CDISC (SDTM and ADaM) standards.
Comprehensive clinical and regulatory biostatistics and statistical programming services in all phases of drug and device development.
Pepgra offers regulatory biostatistics and statistical programming services as per International Conference on Harmonization (ICH) E9 guidelines. We have a thorough understanding of the science of diseases and compounds; this know-how equips us to provide comprehensive planning and assistance.
We offer advanced CRO biostatistical capabilities with solid experience in a wide range of therapeutics and complex study designs (e.g., adaptive, Bayesian BLRM statistics, especially for medical devices, outcome, depending on switching/crossover biomarker) and the implementation of complex methods in macros (RPFST, IPE, IPCW). Our regulatory biostatisticians have a firm grasp of time-tested and latest statistical methods to give you an accurate evaluation of scientific data with experience in handling all phases of drug development and medical device clinical studies in every therapeutic area.
Our regulatory biostatisticians and programmers are an integral part of the assessment process as we perform regular training sessions on relevant topics such as CDISC (SDTM, ADaM), ICH and agency guidelines (e.g. FDA, Section 513(a) of the Federal Food, Drug and Cosmetic Act (FFDCA) mandates the Bayesian approach as the least burdensome appropriate means of evaluating effectiveness of a device). Therefore, we guarantee that the statistical principles outlined in the ICH E9 are in conformation when we analyze and generate datasets, tables, listings, and graphs clinical trial data for submission to regulatory authorities. We adhere to QC phases: 1) validation of programming specifications, 2) code review and validation, 3) data quality checks, and 4) standard QC checklists.
Researchers develop innovative methodologies and analytical techniques using SAS and R Programming. We join hands with the best in the field of health and medicine to provide efficient and cost-effective analysis related to neuroimaging, cardiac and pulmonary diseases, and many other clinical disciplines. We customize services to meet your requirements and thus add value to the time and money you invest in us.
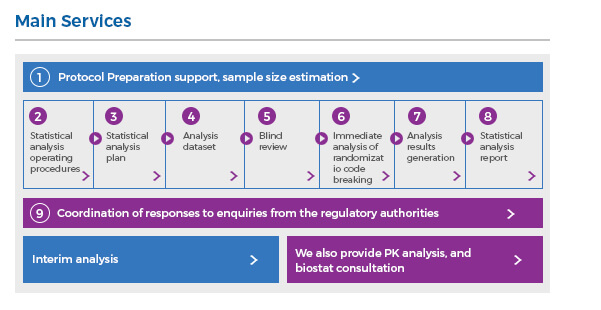
Comprehensive Biostatistics and Statistical Programming Solutions
The Biostatistics division has extensive experience in reporting research findings. The scope of our statistics starts spans requirements gathering to insightful reports for decision making. Work with us for your biostatistical requirements and free your human resources to focus on important things.
We deliver study designs balanced to meet your business needs and expectations with the current scientific understanding and all regulatory requirements considered.
Allow us to help propel your product forward.
Pepgra’s processes are on par with Clinical Data Interchange Standards Consortium (CDISC) principles. They ensure thoroughness at each phase and report accurate findings. Face your uncertainties with the help of their statisticians.
— Dave Miller, CEO of leading drug manufacturer.
We’ll scale
up as your needs grow.
No compromising on integrity and quality. Our processes are well defined and flexible to ramp up as per your requirements.
Partnering with
you till the project end.
We come with you all the way. From design to market support
Pepgra CRO Offerings
"Changing global regulatory system, globalization of clinical trials, increased consumer expectations, infrastructural and culture issues, and various diagnostic requirements should never hamper your research and development programs. With our support..."
Download brochure on our CRO offerings (PDF).



