Clinical Study Design
Services
- Clinical Trial Audit & Monitoring
- Clinical Study Design
- Global Regulatory & Clinical Writing
- Clinical Biostatistics
- Clinical Trial Patient Recruitment
- Regulatory Affairs
- Clinical Data Management
- Post-Market Surveillance
- Clinical Technology & Process
- Healthcare Analytics
- Global and Local Literature Search Screening
Biomedical research study
Pepgra is your reliable CRO. Our experts in scientific, clinical, regulatory, and statistical study will design your clinical protocols and execute trials.
Clinical study design and protocols that balance the interests of multiple stakeholders
To estimate the magnitude of treatment effect or difference in the treatment effect, there is a need for a good trial design approach that allows the treatment effect to be sorted out from person-to-person with variation in the responses. With a better-informed protocol, clinical trials can answer the objectives of the study while reducing the bias in estimating treatment effects thereby reducing the overall time, costs and also increase value to stakeholders.
At Pepgra, we are sensitive to the need of balancing scientific, regulatory and logistical concerns to design and plan a successful clinical investigation. Our team has experience in developing study design for both new drugs and medical device despite the risk stratification (or class) of the device. Our experts develop study design based on strong scientific evidence, and our study design experts regularly advise on the necessity, implications, and practicality of clinical study design factors.
Clinical Study Design Solutions
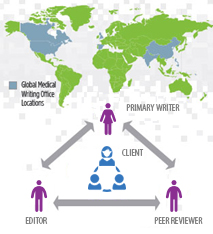
Pepgra has done plethora of work in the area of clinical study design for top pharmaceutical companies. Our clinical experts will evaluate the efficacy of drugs and procedures while satisfying all stakeholders.
We deliver study designs balanced to meet your business needs and expectations with the current scientific understanding and all regulatory requirements considered.
Allow us to help propel your product forward.
Three years ago, we had roped in Pepgra to do the clinical study design; in retrospect, we don’t regret it. They took over right from design and protocols to our submission of IDE to FDA. Their Case Report Form (CRF) was impeccable and so were all their assignments—on par with regulatory standards. If you have to make a choice of a CRO partner, then you can bet on Pepgra.
— Martha Evans, Senior Vice President of a leading pharma company.
We’ll scale
up as your needs grow.
No compromising on integrity and quality. Our processes are well defined and flexible to ramp up as per your requirements.
Partnering with
you till the project end.
We come with you all the way. From design to market support
Pepgra CRO Offerings
"Changing global regulatory system, globalization of clinical trials, increased consumer expectations, infrastructural and culture issues, and various diagnostic requirements should never hamper your research and development programs. With our support..."
Download brochure on our CRO offerings (PDF).



